Company Profile
![]()
|
|
|
|
|
in April, 1951 |
|
|
Tokyo, Japan |
|
|
1. Saitama Factory 2. Midorigaoka Factory 3. Fujioka Factory |
|
|
175 |
|
|
J.Yen 4894 million (March 2025) |
Product Lines
![]()
|
|
5% |
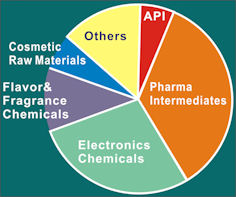
|
|
30% |
|
|
(Photoinitiators, Liquid Crystals) |
35% |
|
|
|
10% |
|
|
|
5% |
|
|
|
15% |
Custom Synthesis & Contract Manufacturing
![]()
|
|
|
Pilot-Scale(10-100kg)custom synthesis Spot-basis custom synthesis (any quantities) Commercial production in custom synthesis |
|
|
|
|
|
temp., at -90℃ to 250℃, in 50-4,000L of volume |
History
![]()
|
1951 |
Midori Laboratory was founded by Masao Horie and started synthesis of flavor & fragrance chemicals. |
|
1962 |
Reorganized as ‘Midori Kagaku Co., Ltd.’ Saitama Factory started operation. |
1977 |
Fukushima Factory was constructed and started operation to expand capability of High-Pressure hydrogenation and distillations. |
1984 |
Okuma Factory was newly constructed and started operation, with computer controlled Multi-Purpose reactors. |
1990 |
In Saitama Factory, GMP facility was improved for production of API and pharmaceutical intermediates. |
|
2004 |
Tokyo Head Office building was newly constructed in Toshima-ku, Tokyo. |
|
2011.3 |
In Okuma Factory and Fukushima Factory, operations are being ceased since the earthquake on March 11, 2011. |
|
2011.11 |
Midorigaoka Factory was constructed and started operation in November 2011 for production of flavor & fragrance chemicals and organic intermediates. |
|
2017.1 |
Fujiokaoka Factory(66,000m2), Gunma pref., was newly constructed and started operation in January, 2017. |
|
2019.2 |
Fujiokaoka Factory A1, A2 and A6 facilities were newly constructed and started operation. |
|
2019.2 |
Fujiokaoka Factory was approved as a pharmaceutical production facility by the authority. |
|
2022.1 |
Fujiokaoka Factory A5 facility and R&D laboratories were newly constructed and started operation. |
|
| Greeting | General Information | Site Location | Product Lines | | Production Plant Equipment | Typical Reactions | Product List ( Fine Chemicals / Photoinitiators ) | |
|
| Home | |